Up to 300,000 women are thought to have been prescribed Stilbestrol over four decades, as ITV News Social Affairs Correspondent Sarah Corker reports
ITV News has discovered new evidence that a dangerous drug linked to cancer was given to mothers nearly a decade after it was supposed to have been banned.
Now, in a major development, the Health Secretary Wes Streeting confirmed the government is considering enhanced cancer screening for those impacted by the use of Stilbestrol, also known as DES, and has vowed to “look seriously at these allegations.”
What is DES?
Stilbestrol, also known as DES, was prescribed on the NHS to prevent miscarriage and to stop breast milk production from 1939 until the late 1970s.
Marketed as a ‘wonder drug’ – a synthetic form of female hormone oestrogen – it has become one of the biggest drug disasters in the NHS’s history.
ITV News can reveal that doctors, regulators, and successive governments failed to act and protect women from the dangers.
Other countries around the world, such as the United States, banned the drug in the 1970s as scientific studies linked the use of DES with breast, cervical, and vaginal cancers. In the UK, health authorities failed to do the same.
The UK government claimed that in 1973, a letter was sent to all doctors telling them to stop using DES for pre-menopausal women, but ITV News has found dozens of women who say they were given it after that date, some as late as 1980.
Susan Miller, 73, from London, believes she was given the drug in 1975 after the birth of her daughter to stop her breast milk – that is two years after the government said GPs were told to stop prescribing the drug.
She recalls questioning the doctor about the drug’s side effects whilst on the maternity ward, but told ITV News those concerns were dismissed.
“I was lied to. It’s absolutely disgusting. I should have never been given the drug. It’s ruined so many people’s lives.”
It’s estimated that up to 300,000 women were prescribed Stilbestrol over four decades. Mrs Miller is among more than 200 people who have contacted ITV News after seeing our ongoing DES investigation.
“It’s not just me, it’s other women as well. They are walking around with time bombs in their breasts, because they don’t even know, so they can’t even get checked,” she said.
The mother of one believes the effect on her health has been devastating. She’s survived blood cancer but now has an aggressive form of breast cancer and is undergoing treatment.
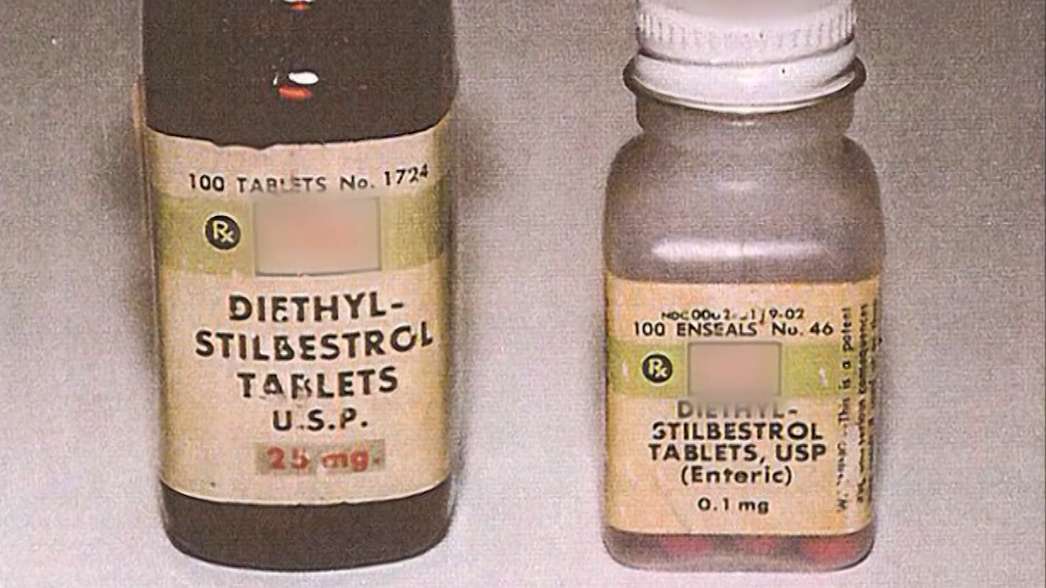
Research suggests that DES mothers may have a 30% higher risk of breast cancer.
If the drug was taken while pregnant, the harm can be passed down through the generations. Daughters exposed in the womb are at increased risk of clear cell cancer of the cervix and vagina and reproductive abnormalities.
Despite the known increased risks, successive governments have failed to introduce enhanced screening, which women say would be ‘lifesaving.’
ITV News has also spoken to former midwives who recall administering DES on maternity wards as late as 1979, and doctors who later treated women with aggressive forms of cancer which have since been linked to DES exposure.
‘Massive regulatory failure’
In 1971, US scientists proved DES was unsafe for use on pre-menopausal women. The medicines watchdog, the MHRA, repeatedly told ITV News that in May 1973, “the Committee on Safety of Medicines wrote to all doctors to advise against the use of DES in pregnancy and women who have not yet gone through menopause.”
No evidence of that letter can be found. A series of Freedom of Information requests and internal reviews from ITV News to the MHRA were rejected.
Our team has searched through hundreds of pages of public health records at the British Library and National Archives, and there is no evidence of that 1973 letter.
In fact, there is no evidence to show that DES was withdrawn or restricted, despite mounting evidence of the drug’s sinister side effects.
Dr Sonia Macleod, from Oxford University and an expert on pharmaceutical safety, said, “There are clear indications that more could and should have been done by the regulators at the time, and if you look at it in this way, that becomes a regulatory failure.”
Dr Macleod believes the government bears ultimate responsibility for the impact on women.
“I think women have been hugely failed in the UK, and particularly because this was a drug that was developed through government funding,” she said.
“There must be accountability and responsibility. Compensation should come from the government. The impacts are horrendous and have been ignored and unseen. It is so wrong,” she said.
Dr Sonia Macleod, from Oxford University and an expert on pharmaceutical safety.
On the south coast in Bognor Regis, Mary Jarman believes she was given DES in 1977, years after warnings about the drug.
Then aged 19, she was prescribed the pills by her GP to stop her breast milk after giving birth prematurely. Ms Jarman later suffered a severe reaction, resulting in emergency breast surgery.
“It was a drug that nobody should have had, and they realised what it was doing, they should have stopped it. But I think because I had an old family doctor, they just kept handing it out,” she said.
Decades later, in her 40s, she developed cervical cancer and had a full hysterectomy.
“If that has caused all the trouble, now I can understand I wasn’t just unlucky to have all those women’s problems, it was all connected.”
Mary Jarman believes she was given DES in 1977, years after warnings about the drug.
Poor NHS record keeping and the casual way DES was given out means women may never know for sure what they were exposed to or the long-term impact it has had.
There are growing calls for a nationwide investigation. There has still been no attempt to trace and inform those exposed to this dangerous drug, and limited research into the long-term health implications.
While thousands of DES victims have sued pharmaceutical companies in the US, France, and the Netherlands, there have been no successful cases in the UK.
In response to our investigation, Dr Alison Cave, MHRA Chief Safety Officer, said: “We express our sympathies with those harmed by the historic use of Diethylstilbestrol (DES).
“We are continuing to invest significant resources to locate historical documentation relating to regulatory decisions on DES made in the 1970s, over 50 years ago. Due to the age and format of the records, this is a complex and time-consuming process.
“We are living now in a different regulatory era….Today, the requirement for patients to be directly provided with information about their medicine is underpinned by legislation.”
A Department of Health and Social Care spokesperson said the Secretary of State has been clear that he will look seriously at these allegations.
Health Secretary, Wes Streeting.
For more information or support about the issues raised in this report, visit:
- Information on Diethylstilbestrol (DES) Exposure and Cancer
- There is guidance on the UK Government website about cervical screenings
- Legal firm Broudie Jackson Canter has set up a campaign page for those affected here
- The NHS website has information on cervical cancer here
- The NHS website has information on breast cancer here
- If you think you need medical help right now, please contact your GP or 111 online can tell you what to do next
Have you been impacted?
If you or anyone you know has been affected by this issue or has a story to share, get in touch
Follow STV News on WhatsApp
Scan the QR code on your mobile device for all the latest news from around the country




























