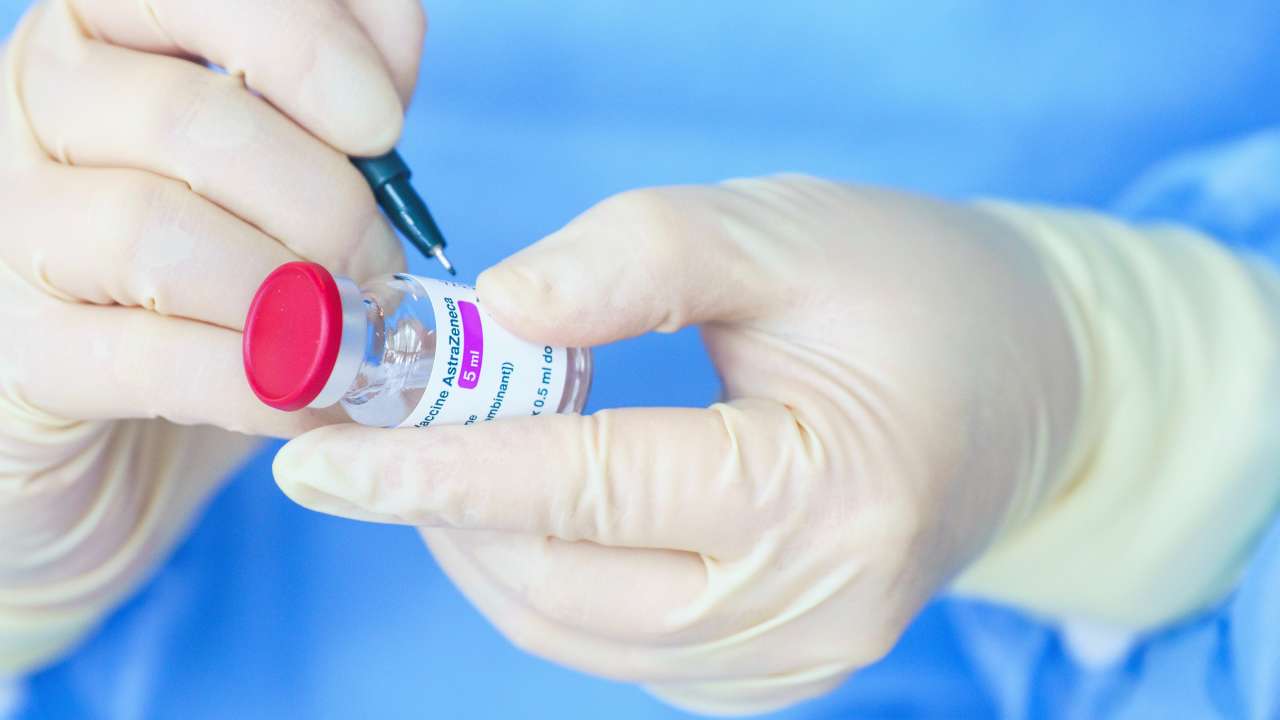
AstraZeneca has begun a worldwide withdrawal of its Covid-19 vaccine due to a surplus of other options targeted at new variants.
The announcement comes months after the British-Swedish drugmaker voluntarily withdrew its EU marketing authorisation for the vaccine, branded Vaxzevria, amid increased scrutiny over blood clots that could be caused by its jab.
AstraZeneca said: “As multiple variant Covid-19 vaccines have since been developed there’s a surplus of available updated vaccines.”
The company said the drug was no longer being manufactured or supplied.
Although the vaccine was found to be safe by countries across the world, it carried the risk of extremely rare side effects by causing Thrombocytopenia Syndrome, which causes potentially lethal blood clots.
Studies estimate around two-three people per 100,000 who were injected with the vaccine developed blood clots.

The World Health Organization also said that the vaccine could have fatal side effects.
They said: “A very rare adverse event called Thrombosis with Thrombocytopenia Syndrome, involving unusual and severe blood clotting events associated with low platelet counts, has been reported after vaccination with this vaccine.”
In the UK, around 325 people died per 100,000 infected with Covid-19, according to Johns Hopkins University.
On April 7, 2021, the Medicines and Healthcare Products Regulatory Agency (MHRA) issued updated information on the “possible risk of extremely rare and unlikely to occur specific types of blood clots” following vaccination with the AstraZeneca jab.
The regulator said the benefits of vaccination “continue to outweigh any risks” but advised “careful consideration be given to people who are at higher risk of specific types of blood clots because of their medical condition”.
The vaccine, often referred to colloquially as the Oxford vaccine due to AstraZeneca’s partnership with Oxford University, played a pivotal role in bringing an end to the pandemic.
Although the Pfizer vaccine was approved first, its extremely high cost and requirement to be kept at very cold temperatures made it only attractive to richer countries who could accommodate it.
The AstraZeneca vaccine was much cheaper and easier to handle and was used across the world as a cheap alternative, with 3bn (enough for 1.5bn people) of the doses supplied globally.
AstraZeneca says their vaccine saved the lives of 6.5 million people in its first year of production.
Follow STV News on WhatsApp
Scan the QR code on your mobile device for all the latest news from around the country