A man with an incurable form of bone marrow cancer has hailed a ground-breaking new lifeline drug approved by the NHS.
The two drugs, elranatamab and teclistamab, will be available for people with myeloma in Scotland who have received three treatments or more.
The condition affects around 24,000 people in the UK every year with more than 3,000 deaths to the disease annually.
Graeme Caulfield, 54, said he thought he had lost all hope when he was offered the new treatment by doctors in February.
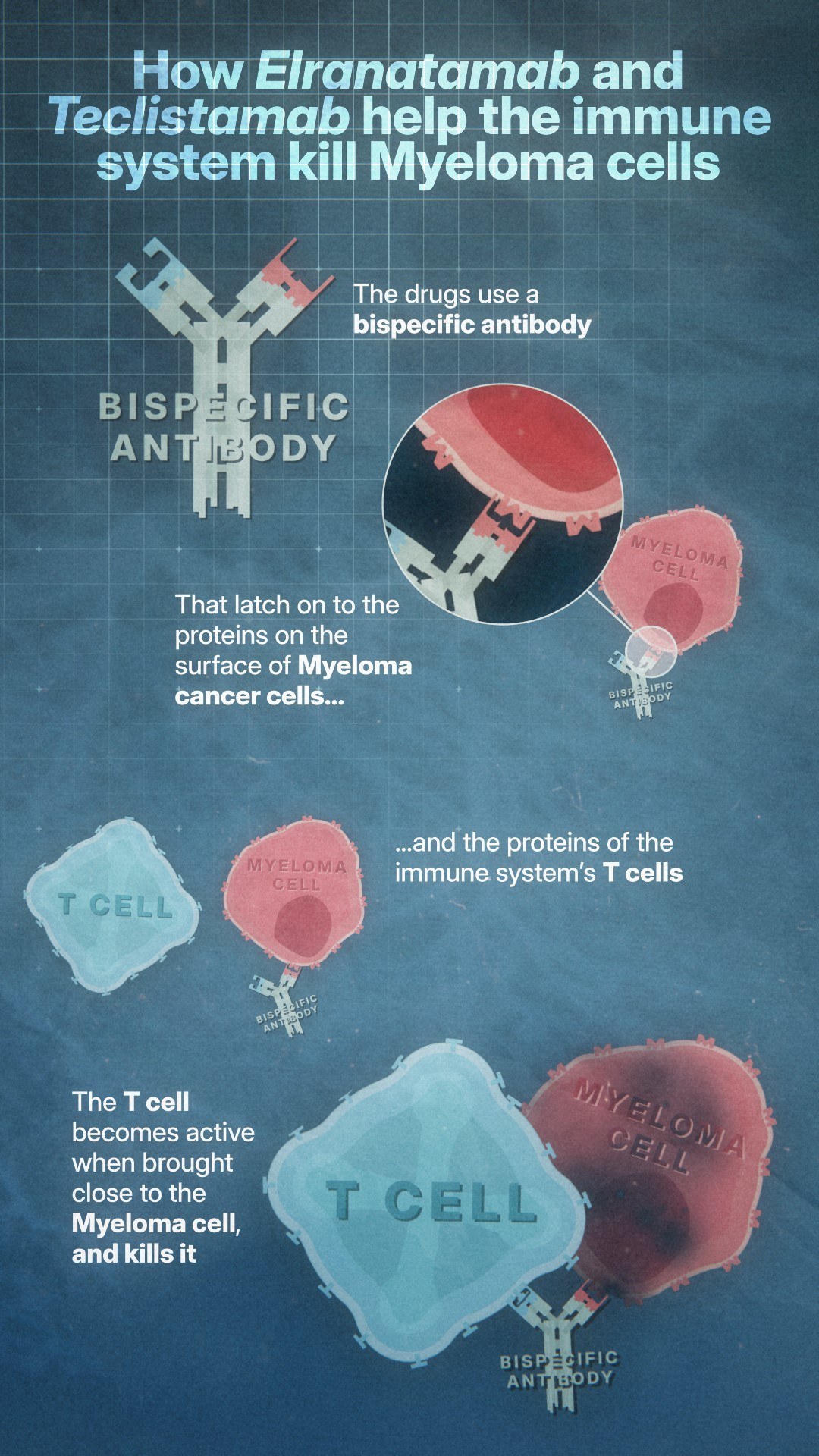
Known as bispecific antibodies, the pioneering treatments are the first new class of myeloma drugs to be approved in Scotland in nearly seven years.
They’ve both been shown to stop myeloma in its tracks for a minimum of 11 months on average and even allow some people who had never fully responded to treatment up to that point to get their first complete remission.
Graeme Caulfield was diagnosed with myeloma after being rushed to hospital with kidney failure in October 2014.
 STV News
STV NewsHe is among the first in Scotland to try the “life-changing” drug in the form of a weekly injection and is now currently in a period of remission.
He told STV News: “I call it a wonder drug. It’s like a dream. I’d take this all day very day. I’d be delighted to keep plugging away with this health.
“I’d ran out of treatments in February – at that stage I felt it was the end of the line for me. I thought I’d possibly go home and survive three months. How do you focus on that? Unfortunately for me, that was the chat.
“I would say we noticed a different after a couple of months, watching the counts go south. Hopefully it prolongs for a long time.
“For me, there is hope that there is something out there.”
On average, 16 people are diagnosed with myeloma every day in the UK.
Despite being the third most common type of blood cancer, myeloma is especially difficult to detect as symptoms, chief among them pain, easily broken bones, fatigue and recurring infection, are often linked to general ageing or minor conditions.
Half of patients face a wait of over five months to receive the right diagnosis and around a third are diagnosed through an emergency route.
While it is incurable, myeloma is treatable in the majority of cases.
Half of all myeloma patients will survive their disease for five years or more, while around one third of myeloma patients will survive their disease for ten years or more.
Graeme recalled the devastating moment he was told by doctors he had multiple myeloma a week after entering the renal ward at the Western Infirmary in Glasgow ten years ago.
He added: “I was a fit, young guy and it was new to me. I was quite active but I had been feeling very fatigued.
“I thought ‘here we go.’ I started treatment four days later. It was a bit of a shock.
“You think ‘it can’t be me.’ The journey has been a massive rollercoaster.”
 STV News
STV NewsTreatment for myeloma aims to control the disease, relieve the complications and symptoms it causes, increase patients’ life expectancy and improve their quality of life.
Teclistamab and elranatamab’s pioneering mechanism of action helps the immune system to recognise and kill cancer cells by binding to both myeloma cells and to T cells.
T cells are a type of white blood cell in our immune system. When the bispecific antibody brings them into close contact with the myeloma cells, they are able to destroy these cells.
Graeme has to take antibiotics and painkillers to manage symptoms including fatigue, bone pain and nerve damage as a result of his condition – but he said it’s the best he’s felt in years.
He said: “I had reported a lot of unusual back pain at the time and I spoke to the pharmacist – he said ‘that’s brilliant, that’s the treatment fighting the cancer cells’.
“It’s absolutely outstanding. I couldn’t believe the results I was getting. A small injection goes a long way.
“It’s a lot of treatments in ten years but this could be the one. You keep fighting.”
Graeme said the support of his family and friends and the hard work of NHS staff has helped keep his spirits up throughout his journey.
 STV News
STV NewsHe said: “The hand-picked angels have done incredible to get me sitting here. They always fix me up.
“Hopefully we can prolong and keep me here. My family and friends have been amazing. The support I’ve had is incredible.
“There’s a lot of people you see at the clinic. Over the last ten years I’ve watched to hopefully see the same person come round. You see people and think ‘they’re still going.’
“There are new treatments coming through to give people a good quality of life.
“When I’m sleeping, someone is working on another treatment behind the scenes.
“I hope it lifts someone’s spirits to see the good results I got. When your door gets chapped with incurable cancer, your reality is here. You’re forced to slow down.
“My prayers were answered. If this one fails, there could be something again. Never give up.”
Elranatamab and teclistamab are aimed at people at fourth line and beyond, who have previously received an immunomodulatory agent such as lenalidomide (Revlimid®), a proteosome inhibitor such as bortezomib (Velcade®) and an anti-CD38 antibody like daratumumab (Darzalex®).
 STV News
STV NewsAccording to the latest data from the MajesTEC-1 trial, patients’ overall response rate to teclistamab is 63%.
Meanwhile, the MagnetisMM-3 trial has shown an overall response rate to elranatamab of 61%.
While teclistamab was approved permanently on the NHS, elranatamab was granted interim approval.
This means that elranatamab is currently approved for use because the Scottish Medicines Consortium recognises the benefits and cost-effectiveness of the treatment for patients, but that the drug will be reassessed later, when ongoing clinical trials provide more information about aspects like long-term effectiveness.
Myeloma UK confirmed it will monitor the situation and make the case for permanent access to elranatamab.
Chiefs at the charity say it’s a “huge day” for the myeloma community, which could help patients to live “longer, happier lives.”
Shelagh McKinlay, director of research and advocacy at blood cancer charity Myeloma UK, said: “We’re absolutely delighted. Elranatamab and teclistamab are the first new class of drugs to be approved in Scotland in seven years and each could be a lifeline for people who are close to running out of treatment options.
“They have both shown excellent results in clinical trials and allowed some people who have never responded well to treatment to experience their very first complete remission.
“Until we have a cure, it is absolutely vital that all myeloma patients are given as many options to tackle their cancer as possible – no matter where they are on their treatment journey.”
Follow STV News on WhatsApp
Scan the QR code on your mobile device for all the latest news from around the country




























