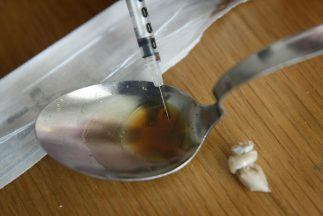
A new treatment for addicts where they are given implants that last six months to help wean them off drugs such as heroin and morphine has just been approved for use in the NHS in Scotland.
The Scottish Medicines Consortium (SMC) has backed the use of buprenorphine implants, saying that with other help, such as psychological treatment, these can help people “focus on recovery”.
While drug users have been prescribed the heroin substitute methadone, they have often had to make daily trips to the pharmacy to get this.
But buprenorphine implants, which are inserted into the arm in a minor surgical procedure, can last for six months, with SMC chairman Mark MacGregor saying they “may be a useful option”.
As well as approving these for use by the NHS in Scotland, the SMC backed new drugs for treating cancer patients.
Olaparib was accepted for the treatment of some relatively rare and aggressive forms of ovarian cancer.
The drug, when administered with another treatment bevacizumab, can extend the time patients have before the disease progresses, preventing a worsening of symptoms and delaying the need for further chemotherapy.
SMC also approved ibrutinib (Imbruvica) as a treatment for those suffering from Waldenstrom’s macroglobulinaemia, a rare and incurable cancer of white blood cells.
Patients with this disease can suffer extreme fatigue, recurrent infections, pain and breathlessness, with the new treatment a better option for them as it comes in tablets which can be taken at home, reducing the number of hospital visits they need to make.
In addition to this the drug nivolumab was approved for the treatment of bowel cancers with a specific genetic mutation which have spread.
The drug is used alongside another medicine, ipilimumab and together these can work to stimulate the immune system to fight the cancer, which can help some patients live longer.
Cancer Research UK welcomed the approval of more treatments, with David Ferguson, public affairs manager for the charity in Scotland, saying: “It’s great news for patients that olaparib and nivolumab have been approved for further use in NHS Scotland.
“Cancer Research UK played a leading role in the first clinical trials for olaparib, which led to it being used routinely to treat ovarian cancer in patients who have the BRCA1 and BRCA2 gene faults.
“Olaparib is now a well-established treatment option for ovarian cancer and today’s decision reaffirms that position for advanced ovarian cancer.”
He continued: “Nivolumab provides a more targeted approach to treating cancer, by helping the immune system to attack tumours.
“Studies in metastatic colorectal cancer have found that it can help some patients live longer, as well as producing fewer side effects than other treatments.
“The approval of ibrutinib represents a major step-change for patients with Waldenstrom’s macroglobulinaemia, as it offers a new treatment option for this rare blood cancer.
“It has shown great promise in clinical trials with patients whose cancer has come back after previous treatment, helping to keep cancer at bay for longer.
“We hope that today’s approvals will give patients facing these advanced forms of cancer better options for treatment, to help them make more memories with the people they love.”
Follow STV News on WhatsApp
Scan the QR code on your mobile device for all the latest news from around the country


 PA Media
PA Media