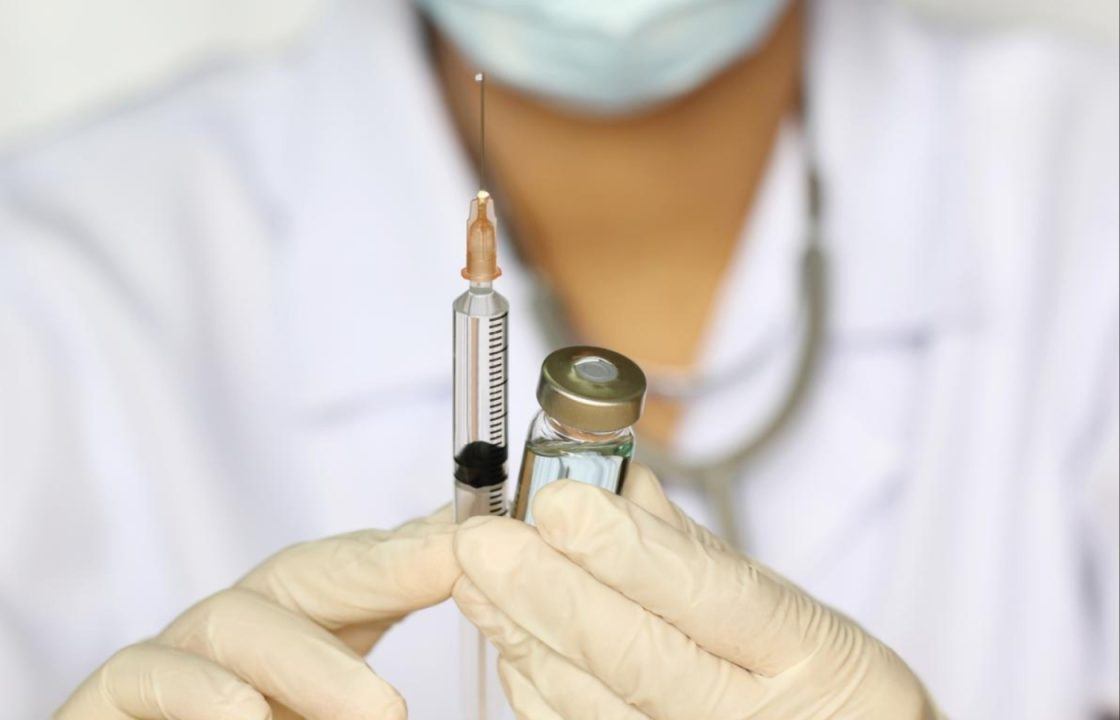
Women are being called up to take part in a trial to test the effectiveness of a six-monthly contraceptive injection by researchers at a leading Scottish university.
The trials are set to commence in the Lothians and Ayrshire and, if successful, could lead to an expansion of the range of contraceptive choices available for women.
The University of Edinburgh-led pilot will be part of a global study building on previous research.
Some 750 volunteers between the ages of 18 to 35 will be needed to take part in the study across four countries.
For those looking to sign up, participants will be examined and have their blood tested before taking part.
All participants will have scheduled follow up visits to check their progress for the full 13-month study duration.
Volunteers will also be required to undertake pregnancy tests each month, with a check-in phone call to follow up on results.
The trial uses a contraceptive injection of depot medroxyprogesterone acetate (DMPA), similar to the existing injectable method.
Women will receive an injection of DMPA under the skin every six-months.
The contraceptive injection is used by 15% of women in the UK, and currently involves injecting DMPA into the muscle on a three-month basis.
DMPA contains the hormone progestogen, which acts to prevent pregnancy by stopping the release of the egg from the ovary.
It also thickens the cervical mucus, which makes it difficult for sperm to access the womb.
If success, researchers say the new method could be more convenient with women only required to attend two appointments a year, instead of four currently needed.
It is thought the method could also lead to increased effectiveness as having to inject less frequently should reduce unintended pregnancy due to missed or late injections, experts say.
Current side effects may also be improved as, when injected under the skin instead of into the muscle, the drug is absorbed more slowly resulting in a gentler peak in hormone levels.
Moving to two injections per year would reduce the amount of DMPA injected by half which could also lead to a more rapid return of fertility after stopping the contraceptive and further improvements to side effects, according to scientists.
Previous research of 21 women showed that DMPA stopped all participants from ovulating for at least six months when it was administered under the skin.
People who would like to take part can register their interest by contacting the study team in Edinburgh at Chalmers.Research@ed.ac.uk, or Ayrshire at Clinical_TrialsNursesRD_XH@aapct.scot.nhs.uk
Follow STV News on WhatsApp
Scan the QR code on your mobile device for all the latest news from around the country